|
The
emergence of global business systems, and along with it the need
for protection of Intellectual Property Rights (IPR) are now
almost foregone conclusions. But what caused them? There are at
least two obvious factors that contributed to this changed global
political economy and the concomitant introduction of IPR. By the
early 1970s, the high labor costs in the OECD (Organization for
Economic Cooperation and Development) countries had led to the
relocation of labor intensive industries to third world countries,
which saw the beginning of transition from a manufacturing based
economy to a service based economy dominated by
knowledge-intensive industries.
On the other hand, the condition of indebtedness of the Third
World of the 1970s (because of the twin oil crises of 1973-74 and
1979) created an increased demand for foreign investments. Both
these factors fed on each other, resulting in an increased
prominence of transnational companies (TNCs) in the global trade.
For the latter, it was clear that while this situation could lead
to higher revenues, the strategic route lay in enforcing a strict
IPR regime.
In the eighth round of GATT talks (1986-1993), initial attempts by
the United States to introduce a Trade Related Intellectual
Property Rights (TRIPS) regime brought forth strong objections
from the third world countries like India and Brazil. Their
apprehension was that the technology they needed from the west for
development would become more expensive under an active TRIPS
regime. However, in 1988 a compromise was arrived at with certain
trade concessions made by first world countries.
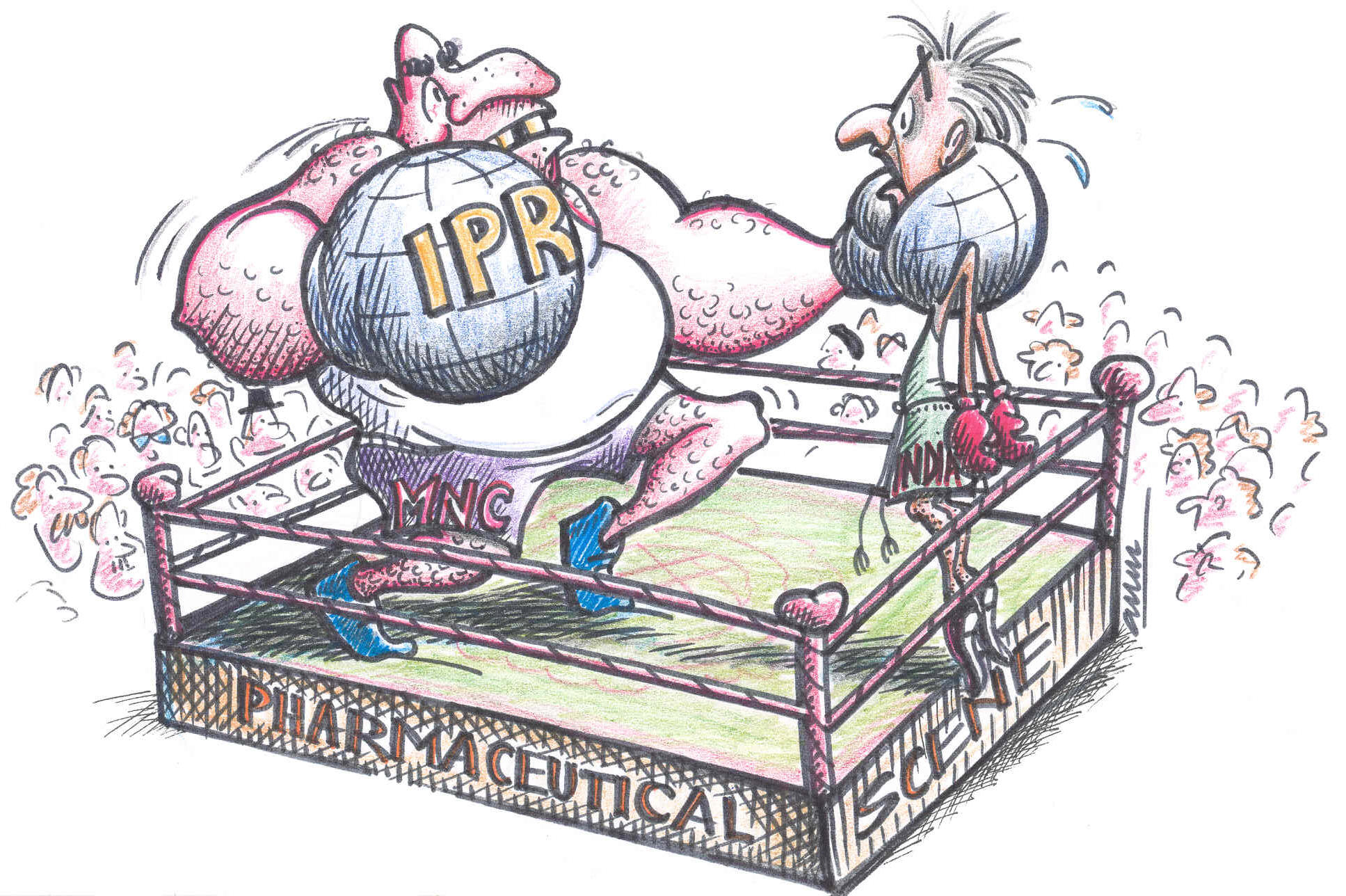
For a country like India, the sector that is likely to be affected
strongly by an IPR regime, (more particularly with respect to
product patenting), is the pharmaceutical industry. The impact may
be felt either through direct competition or via patenting of
Indian tropical bio-diversity by transnational companies. Therefore,
an urgent need for policies and strategies to combat the
situation.
India has made significant strides in terms of
self-reliance in pharmaceutical products. From being an
importer of medicines in the first few decades after independence,
today we are able to meet a good part of our essential requirement
through indigenous production. Prices of indigenously manufactured
drugs, once among the highest, are among the lowest in the world
today. A net foreign exchange earner, with the annual turnover of Rs. 15,000 crores, the Indian pharmaceutical industry today ranks
among the most developed in the third world, in terms of
technology, quality and range of pharmaceuticals manufactured. The
annual increase in the drug production is about 15% , while the
average pre-tax profits for the industry is 8% of the annual
sales. Nevertheless, the share of Indian pharmaceutical business
in the present world market is only 1%, which suggests
considerable opportunities for growth.
The reason for the low price of Indian drugs is twofold. First, as
a result of a government intervention through the Drug Price
Control Order (DPCO), the prices of all bulk drugs and thousands
of formulations have been controlled so as to make them affordable
to the common man. Secondly, and perhaps more significantly, many
Indian companies – particularly, during the early period of the
industry’s growth - have engaged in reverse engineering, a process
that has allowed quick replication of new drugs at a low cost.
But today with an impending strong IPR regime, the major issue
facing the domestic industry is that of investing in R&D so as to
better compete with international corporations in creating new
drugs. The industry’s R & D expenditure is currently about 2% of
its annual turnover - considerably less than the 6-8% spent by
many corporations in the western world. The cost of pharmaceutical
R&D in the developed world is a steep one - around $250-300
million per annum. In India, however, the expenditure might be
only one third to one fourth of the last figure, primarily because
of the low manpower costs. |
Few Indian companies can afford to spend in excess of Rs.100 crores
in R&D and wait for a decade for pay back. Essentially, a basic
research program demands a high “entry fee”, which only companies
with very deep pockets,
large
business volumes, and an extensive
global operation are positioned to accept!
According to the U.S industry, it loses nearly $1.4 billion
annually from the sale of copied drugs in just four third world
countries including India —which has a $0.92 billion share of the
copying. It is difficult to eschew entirely the truth of this
criticism. The moot point, however, is whether the high R&D costs
borne by TNCs alone can legitimize the imposition of a strong IPR
regime. For, amongst other things, historically most first world
countries have introduced product patents only after their own
pharmaceutical industries became strong enough to challenge
international competition.
It would thus appear that the imposition of a strong IPR regime is
more an attempt by western countries and their pharmaceutical
corporations to consolidate the gains they have made in the last
several decades. Just when Indian pharmaceutical companies are
beginning to acquire strength under the process patent regime they
are being forced to concede the gains to transnationals. Of
course, this need not imply that the Indian sector should be
provided a completely protectionist environment indefinitely;
rather there should be a time frame arrived after due
consultations with the industry. Presently our timeframes are
dictated by the external environment and therefore does not do
justice to our internal concerns.Some of the adverse changes that
could affect the Indian pharmaceutical industry under the IPR
regime are: increased R&D expenditure and hence higher Indian drug
prices, and lower export of drugs because of the inability to use
process patents. Overall, however, the use of IPR will not be an
unmitigated disaster if the industry invests well in basic
research, new product launches, a thrust into global generics
market and licensing-in of products from companies that do not
have a presence in India. There needs to be rapid development of
innovative, non-infringing processes for products going off-patent
internationally and a leveraging of the low cost domestic
manufacturing. In an ironic sense, India has already benefited
from an impending IPR regime. For, with the recent attempts by
western players at patenting age-old Indian botanicals such as
neem, turmeric, jar amla and several others, Indian policy makers
have suddenly woken up to the advantages of patenting our
bio-diversity!
There is no doubt that bio-diversity will increasingly gain
importance in drug development. Developing countries, like India
and Brazil, are the source of an estimated 90% of the world’s
store of biological resources. More than half of the world’s most
frequently prescribed drugs are derived from plants or synthetic
copies of plant chemicals — and this trend is growing. It has been
estimated that even if just a 2% royalty were charged on genetic
resources developed by local innovators in the tropical countries
of the South, the North would owe around $5 billion / year in unpaid
royalties for medicinal plants.
To conclude, the use of IPR in the Indian pharmaceutical industry
must be perceived judiciously in light of its strengths and
weaknesses. The role of the government must be more bold and
imaginative in dealing with the industry’s problems and
opportunities.
|